How Many Resonance Structures Exist For The Formate İon Hco2 ? We answer all your questions in detailed articles and titles. Here are the details.
How Many Resonance Structures Exist For The Formate İon Hco2 ?
How Many Resonance Structures Exist For The Formate İon Hco2 ? HCO 2 – or formation is a derivative and salt of formic acid. Let’s discuss some important facts about the structure of HCO 2 – in detail.
The basic structure of HCO 2 – contains one atom each of hydrogen, carbon and 2 atoms of oxygen. The IUPAC name of this structure is methanoate. HCO has been observed to have a fairly high plasticity 2 molecule, which allows it to adopt various structures.
The observed molecular weight of the formation is about 45.01 g/mol. When formic acid loses one of its protons, formations are formed. In the following paragraphs, we will take a closer look at various properties such as Lewis structure, resonance, acidity, etc.
How to draw HCO 2 – Lewis structure?
How Many Resonance Structures Exist For The Formate İon Hco2 ? By using the Lewis structure concept, it becomes easier to understand the nature of the bond. Let’s understand the method of drawing Lewis structure for HCO 2 – .
1. Determination of the total number of valence electrons
The Lewis structure of HCO 2 – has 18 valence electrons. Hydrogen contributes one electron, carbon has 4, and oxygen contributes 12 electrons (6 electrons each through 2 oxygen atoms). An extra valence electron due to the -1 charge.
2. Determination of the atom with the lowest electronegativity
In this step, it is decided which atom will be placed in the middle. In other words, the atom with the lowest electronegativity is placed in the center of the structure. Among the remaining atoms, the electronegativity of carbon is lower. Therefore, carbon is placed in the center of the structure.
3. Arrangement of electron pairs between atoms
Carbon shares a pair of electrons with hydrogen to form a single bond. As you can see, there are 2 oxygen atoms in the structure. That is, carbon shares a pair of electrons with a carbon and two pairs of electrons with the other carbon atom. An electron is represented by a minus sign outside the structure.
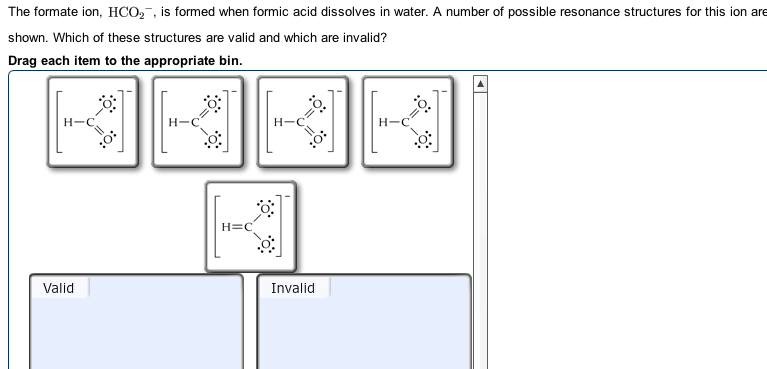
HCO 2 – resonance of the Lewis structure
How Many Resonance Structures Exist For The Formate İon Hco2 ? Resonance is the delocalization of electrons. Let’s talk about the HCO 2 – Lewis structure resonance concept.
Resonance is possible in HCO2 – since oxygen contains lone pairs of electrons capable of delocalizing . Hence there are 2 resonance structures possible for the HCO2 molecule . These resonance structures help us predict the more accurate structure for the molecule.
HCO 2 – Lewis structure form
The type of atoms has a major impact on the shape of the molecule. Let’s discuss this in detail.
The shape of HCO 2 – is trigonal planar . We can see that the central atom is bonded to 3 atoms. And in a trigonal planar geometry, a similar pattern is observed. The atoms in such an arrangement are also called peripheral.
HCO 2 – Lewis structure formal fee
Formal charge is the charge found on atoms that is useful in predicting lower energy. Let’s discuss the formal charges against HCO 2 – .
The formal charge of HCO 2 – Lewis structure is negative one (-1). And that’s because of the second oxygen of the structure.
The formula for calculating the formal fee is
- Formal charge = valence electrons – lone pair of electrons – number of bonds
- Formal charge on hydrogen = 1 – 0 – 2/2 = 0
- Formal charge on coal = 4 – 0 – 8/2 = 0
- Formal charge on the first oxygen = 6 – 4 – 4/2 = 0
- Formal charge on the second oxygen = 6- 6 – 2/2 = -1
Therefore the formal charge as calculated above on HCO 2 – is -1.
HCO 2 – angles of the Lewis structure
How Many Resonance Structures Exist For The Formate İon Hco2 ? In the structure section we discussed that HCO 2 – is trigonal planar. Let’s analyze the bond angle in HCO 2 – .
The bond angle in HCO 2 – is 120°. It has been observed that the hybridization of carbon (the central atom) is sp2 and its steric number is 3. Also, there is no lone pair at the central carbon atom.
HCO 2 – Lewis structure octet rule
Octet rule is the term used to describe the bonding ability of atoms, meaning atoms prefer eight electrons in the outer shell. Let’s see in HCO 2 – .
HCO 2 – satisfies the octet rule. The central carbon atom forms a single bond (sigma bond) with one oxygen and one hydrogen. Forms a double bond with the other oxygen atom by sharing two pairs of electrons.
HCO 2 – Lewis structure lone pairs
Lone pairs in a molecule are the electrons not involved in the covalent bonding of the molecule. Let’s check on HCO 2 – .
There are 5 lone pairs in the molecule. An oxygen of the structure has 3 lone pairs of electrons (oxygen is bound to the central atom with a single bond). The other oxygen has 2 lone pairs of electrons (oxygen bonded to the central atom with a double bond).
HCO 2 – valence electrons
The term is used to refer to the electrons present in the outermost shell of an atom. Let’s discuss for HCO 2 – .
The total valence electrons present in the HCO 2 – Lewis structure is 18. One electron is contributed by the hydrogen atom. Four electrons are contributed by the central carbon atom and 6 valence electrons each by 2 oxygen atoms. An electron due to the charge on the structure.
HCO 2 – hybridization
The mixing of orbitals into a new set of hybrid orbitals is called hybridization. Let’s analyze for HCO 2 – .
The hybridization of the central carbon atom is sp2. The oxygen bond to the central atom with sigma bonding shows sp3 hybridization. The oxygen bond to the central atom with pie bonding exhibits sp2 hybridization.
HCO 2 – solubility
How Many Resonance Structures Exist For The Formate İon Hco2 ? Solubility refers to the degree to which a substance can dissolve in a particular solvent. Let’s discuss for HCO 2 – .
List of compounds in which HCO 2 – is soluble:
- Water 97.2 g/100 ml at 20°C
- ethanol
- formic acid
- glycerin
Is HCO 2 – soluble in water?
Most compounds are water soluble, but at different temperature ranges. Let’s check on HCO 2 –
HCO 2 – is water soluble. The solubility differs at different temperatures. 43 g/100 ml at zero °C and 160 g/100 ml at 100 °C. Water is a universal solvent and has the ability to dissolve almost any compound. We can see that as the temperature increases, the solubility of formation ions also increases.
Is HCO 2 – an electrolyte?
A substance that has the potential to break down in the form of ions and conduct electricity is called an electrolyte. Let’s see if HCO 2 – is an electrolyte.
HCO 2 – is an electrolyte. When the formation comes into contact with aqueous medium, it has the ability to dissociate into an ion. These ions, in turn, can conduct electricity.
Is HCO 2 – a strong electrolyte?
A strong electrolyte is a substance whose ions can completely dissociate into the ionic form. Let’s see how strong electrolyte HCO is 2 – .
HCO 2 – is considered a comparatively weak electrolyte. The reason for this is that its Ka value is around 1.8. (Ka is the dissociation constant, which helps us understand the extent of dissociation) 1.8 is a fairly low value.
Is HCO 2 – acidic or basic?
An acid has the ability to almost completely dissociate compared to a base. Let’s see if HCO 2 – is acidic or basic.
HCO 2 – is the anion of an acid. Formiation is a derivative of formic acid (a weak acid). As we can see the dissociation constant value for HCO 2 – is 1.8 which is usually observed for weak acids. HCO 2 – is an acid, but a weak acid. This is because it is the monocarboxylic acid anion of a weak acid.
Is HCO 2 – a strong acid?
A strong acid has the ability to rapidly and almost completely dissociate into ions. Let’s see how strong Formation is.
HCO 2 – is a weak acid. The reason for its weakly acidic behavior is that it cannot fully dissociate under aqueous conditions.
Is HCO 2 – polyprotic acid?
A polyprotic acid is an acid that can donate many protons under aqueous conditions. Let’s analyze for HCO 2 – .
HCO 2 – is not a polyprotic acid. The reason is that it cannot donate many protons in aqueous solution since it has only one proton in the structure. HCO 2 – is not a polyprotic acid since it is formed when formic acid loses one of its protons. That means there is already a lack of protons.
Is HCO 2 – a Lewis acid?
A Lewis acid is the one that can accept a proton. Let’s check on HCO 2 – .
HCO 2 – is a Lewis acid. The Lewis structure itself indicates this, there is an extra electron in the structure (the minus sign). So a proton can pick it up and complete its structure.
Is HCO 2 – an Arrhenius acid?
How Many Resonance Structures Exist For The Formate İon Hco2 ? Arrhenic acid is a substance that increases the concentration of H+ ions in solution by releasing protons. Let’s check on HCO 2 – .
HCO 2 – is an Arrhenius acid, but not a very strong one. The reason for this is that it only has one proton, which is also not released very easily. Therefore, dissolving formation ions in a solution does not significantly increase the concentration of H+ ions in the solution.
Is HCO 2 – polar or non-polar?
How Many Resonance Structures Exist For The Formate İon Hco2 ? A polar substance has significant charge separation and a non-polar substance has no charge separation. Let’s check on HCO 2 – .
HCO 2 – is a polar molecule, the charges on the molecule are not evenly distributed. That is, there is a significant amount of charge separation in the molecule.
Why and how HCO 2 – is polar?
How Many Resonance Structures Exist For The Formate İon Hco2 ? HCO 2 – is polar because the electronegativity values for oxygen, carbon, and hydrogen are 3.5, 2.2, and 2.5, respectively. If we subtract the electronegativity values for bonds, we will find that there is some difference in the values. Consider the CH bond: the electronegativity difference is 0.3 and for CO 1.0.
Is HCO 2 – linear ?
A linear molecule has a very simple geometry with a bond angle of 180°. Let’s see if HCO 2 – is linear or not.
HCO 2 – is not linear. As already discussed in the sections above, the formation has a trigonal planar geometry and 5 lone pairs. Therefore, the molecule is not able to take on a linear form.
Is HCO 2 – paramagnetic or diamagnetic?
How Many Resonance Structures Exist For The Formate İon Hco2 ? Substances that contain unpaired electrons are called paramagnetic and those without unpaired electrons are called diamagnetic. Let’s analyze for HCO 2 – .
HCO 2 – is paramagnetic. The reason for this is that there is one electron in the structure that remains unpaired (which is written as a minus sign on the structure).
HCO 2 – boiling point
How Many Resonance Structures Exist For The Formate İon Hco2 ? The boiling point for a substance is related to the temperature at which the vapor and atmospheric pressures equalize. Let’s search for HCO 2 – .
The boiling point of HCO 2 – is around 105-109 °C under normal atmospheric conditions (760 mmHg). It can vary with changes in atmospheric conditions.
Is HCO 2 – ionic or covalent?
How Many Resonance Structures Exist For The Formate İon Hco2 ? A covalent bond is formed as a result of sharing electrons between atoms and ions are formed due to electrostatic attraction. Let’s check on HCO 2 – .
HCO 2 – is covalent. The reason for this is that we saw in the section above that bond formation in the molecule occurs as a result of electron sharing.
Hydrogen Bonding in HCO 2 –
How Many Resonance Structures Exist For The Formate İon Hco2 ? Hydrogen bonds form when a proton binds to an electronegative atom. Let’s see if it comes out in HCO 2 – .
Hydrogen bonds are observed in HCO 2 – . There is hydrogen which has the ability to combine intermolecularly with the electronegative atom oxygen present in the structure.
Is HCO 2 – dipole?
How Many Resonance Structures Exist For The Formate İon Hco2 ? A dipole is formed when there is a charge separation in the molecule. Let’s analyze for HCO 2 – .
HCO 2 – has a dipole, since there is separation of charges. The observed dipole moment is about 1.33 Debye.
Is HCO 2 – monoprotic, diprotic or triprotic?
A substance with one proton in the structure is called monoprotic, diuretic has 2 and triprotic has 3. Let’s look at HCO 2 – .
Formation is monoprotic. There is only one proton in the structure of the molecule.
Conclusion
HCO 2 – is a derivative of formic acid. The bond type is covalent with 5 lone pairs of electrons. The observed geometry is trigonal planar with a bond angle of 120°C.
We have conveyed your questions about this subject. Please do not hesitate to comment and ask questions about this topic. Thanks for everything.
How Many Resonance Structures Exist For The Formate İon Hco2 video are below.

